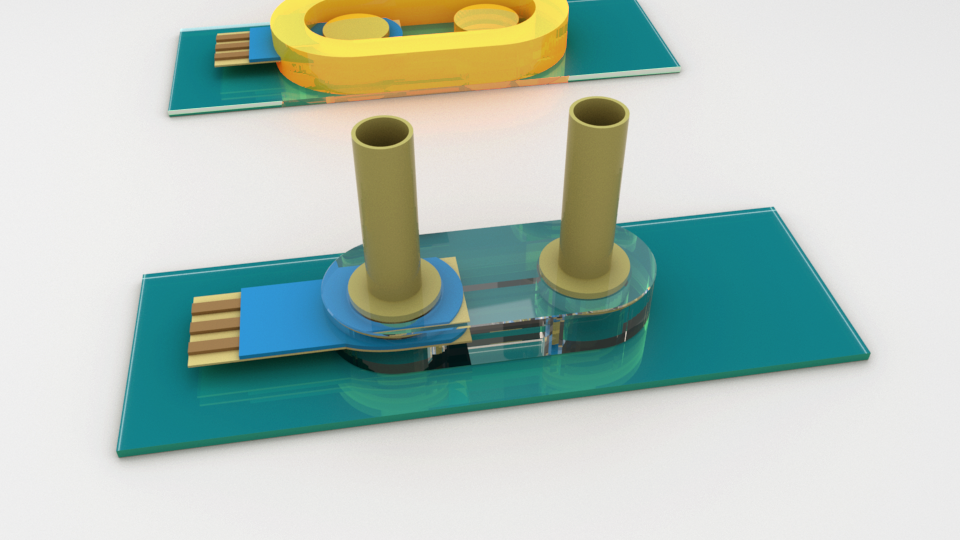
Shaf has been putting enzymes to work in our part of the EPSCoR project, “Powering the Kentucky Bioeconomy for a Sustainable Future.” His goal is to quickly measure the activity of tiny amounts of enzymes supported on membranes. Membranes covered with enzymes can convert one type of chemical to another at room temperature–but it’s unclear which natural or engineered enzymes are the best for a given application. We just have to test them. We’re using hydrogel printing as a gentle method to combine enzyme-coated membranes, electrochemical detection electrodes, and paths for fluid flow and light transmission in a small, disposable package that can measure the productivity of a single enzyme variant. That’s a lot. Good thing we have collaborators (Dr. Anne-Frances Miller at the University of Kentucky, Dr. Mark Running and Dr. Gautam Gupta at the University of Louisville, and students). Their expertise means Shaf is able to get electrochemical and optical detection started on the benchtop, using chemicals that change their conductivity or color when the enzyme acts on them. Meanwhile he’s discovering the best settings to keep the hydrogel printer happy.
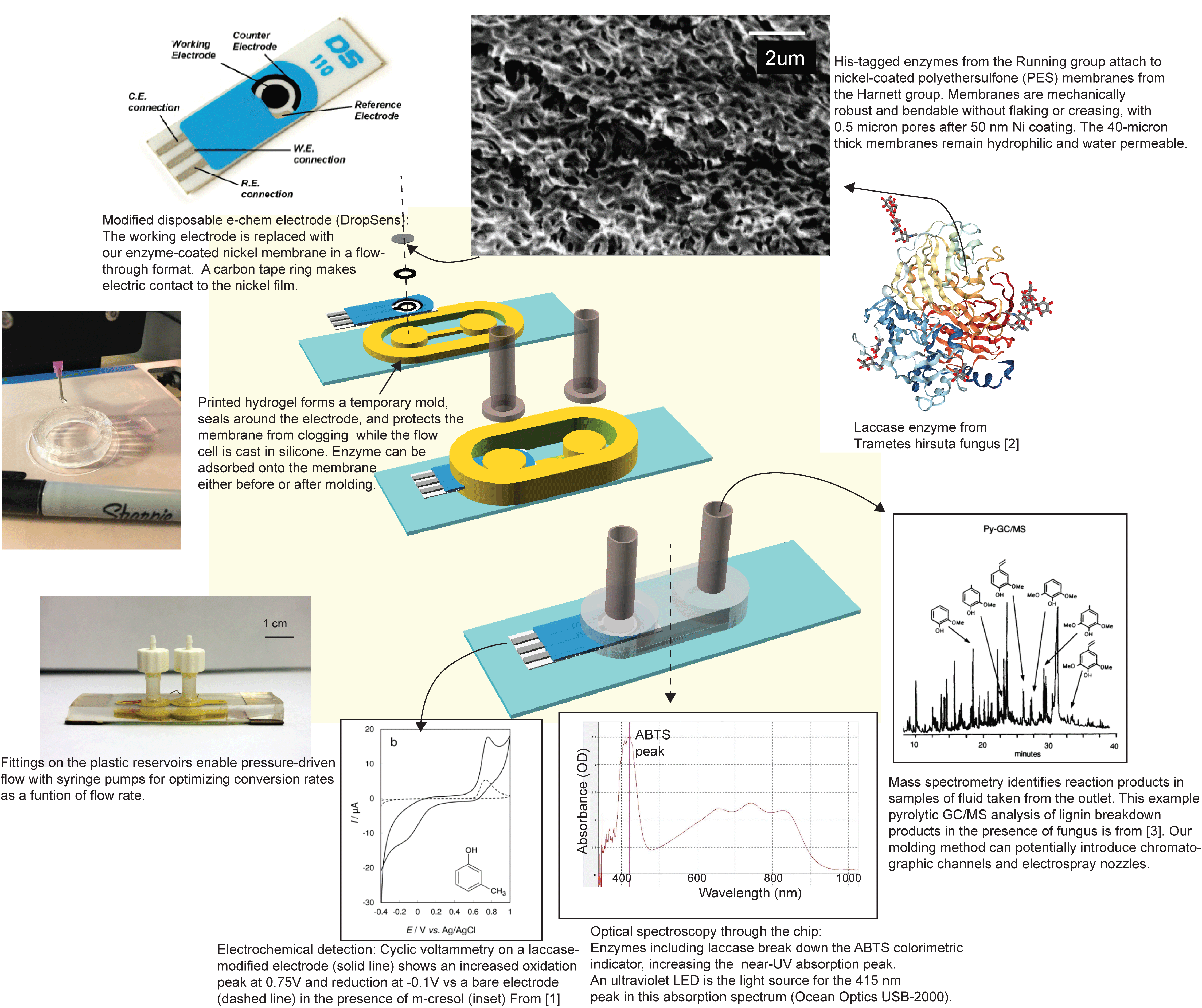
[1] Sýs, Milan, et al. “Electrochemical Study of Trametes Versicolor Laccase Compatibility to Different Polyphenolic Substrates.” Chemosensors 5.1 (2017): 9. APA
[2] RCSB Protein Data Bank ID 3FPX
[3] Martınez, A. T., S. Camarero, A. Gutiérrez, P. Bocchini, and G. C. Galletti. “Studies on wheat lignin degradation by Pleurotus species using analytical pyrolysis.” Journal of Analytical and Applied Pyrolysis 58 (2001): 401-411.